History background on the scientist
Rutherford infomation below
I'm a paragraph. Click me to add your own text and edit me. It’s easy. Just click “Edit Text” or double click me and you can start adding your own content and make changes to the font. I’m a great place for you to let your users know a little more about you. If you want to delete me just click on me and press delete. Click on me to edit me.



Experimental Design/Data collection
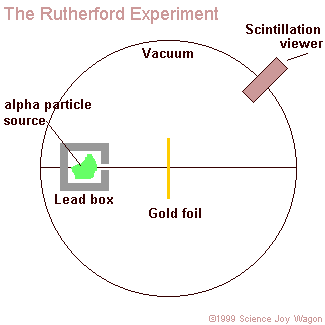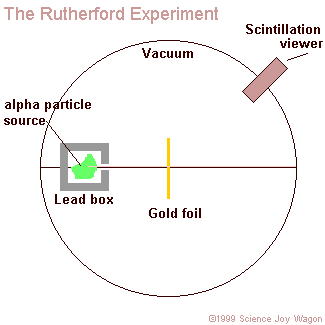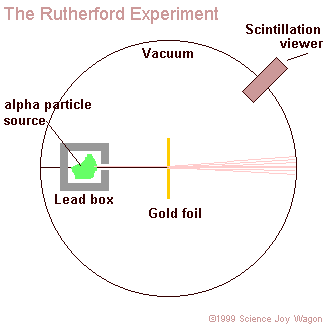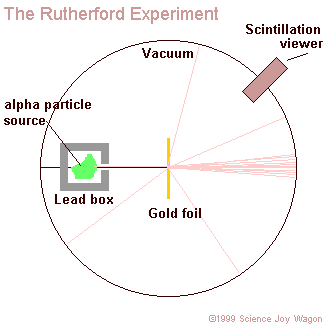
aim a beam of alpha particles at a piece of gold foil that was approximately 8.6 x 10^(-6) centimeters thick. To be more accurate Rutherford actually included a wide variety of different foils (such as: aluminum, iron, and lead), but his use of gold foil is most commonly known. In accordance to the J.J. Thomson model of an atom, the alpha particles should have passed directly through the gold foil for all instances. Therefore to confirm this activity, a zinc sulfide screen was placed behind the foil as a backdrop for the alpha particles to appear upon. Directly above this screen was a microscope that allowed one of the two experimenters (only Geiger and Marsden ) actually performed the experiment, Rutherford just explained the results to observe any contact made between the alpha particles and the screen. In order for the light of the alpha particles to be observed, the experiment was performed in complete darkness. Also, to further enhance the accurateness of the observations the experimenter that was charged with looking through the microscope sat in the dark of the lab room for at least one hour before performing the experiment. This was done in order to allow the experimenter's eyes to reach maximum visual acuity.
- He invented the Rutherford model of the atom, which concluded that the atom contained a very small positively-charged nucleus orbited by electrons.
- He realized that the proton had a positive charge and the neutron had a positive charge, he discovered this by conducting the gold foil experiment.
- Rutherford concluded that atoms have a central positive nucleus surrounded by negative orbiting electrons.
Citation
The Nobel Foundation. (1908). Ernest rutherford - biography. Retrieved from http://www.nobelprize.org/nobel_prizes/chemistry/laureates/1908/rutherford-bio.html
value. quality care. convenience.